BRASILIA/SAO PAULO (Reuters) – Brazil on Tuesday approved clinical trials starting in August for a potential COVID-19 vaccine under joint development by U.S. pharmaceutical company Pfizer and Germany’s BioNTech – the third such vaccine to be tested in the country.
With more than 2.1 million confirmed cases of the novel coronavirus, Brazil has the world’s worst outbreak outside the United States, making it prime testing grounds for vaccines.
“We are proud to have Brazilian volunteers participating in this global effort, which could play a critical role in the fight against COVID-19,” said Edson Moreira, who will lead the Pfizer/BioNTech trial, according to a company statement.
Brazilian health regulator Anvisa had previously approved trials for possible vaccines developed in the UK and China.
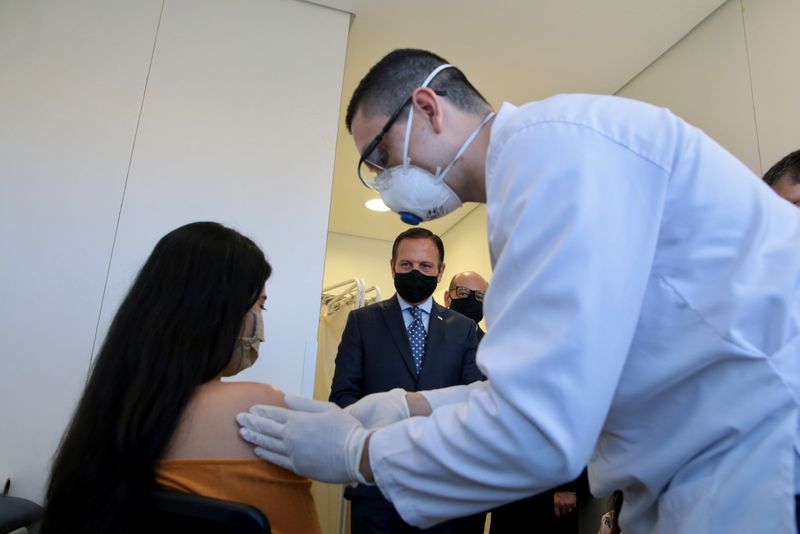
On Tuesday, the first Brazilian volunteer was injected with a possible coronavirus vaccine developed by China’s Sinovac Biotech in Sao Paulo.
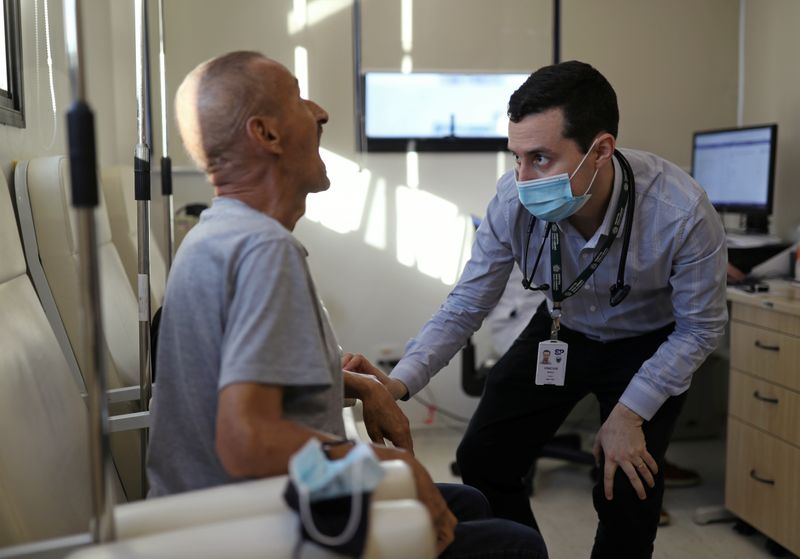
A vaccine developed by the University of Oxford and AstraZeneca entered clinical trials in Brazil in June.
The country is also scrambling to secure supplies of potential vaccines, in case they are proven successful.
Interim Health Minister Eduardo Pazuello said on Tuesday that negotiations are underway with U.S. biotech firm Moderna Inc for Brazil to get priority in purchasing a potential COVID-19 vaccine the company is developing.
Pazuello said Brazil already has an understanding with AstraZeneca for the firm to deliver 30 million doses of its potential vaccine.
Sinovac has agreed to deliver enough vaccine to immunize 60 million Brazilians, if its vaccine is proven effective.
Brazil is also developing possible vaccines domestically, which are still in pre-clinical testing stages.