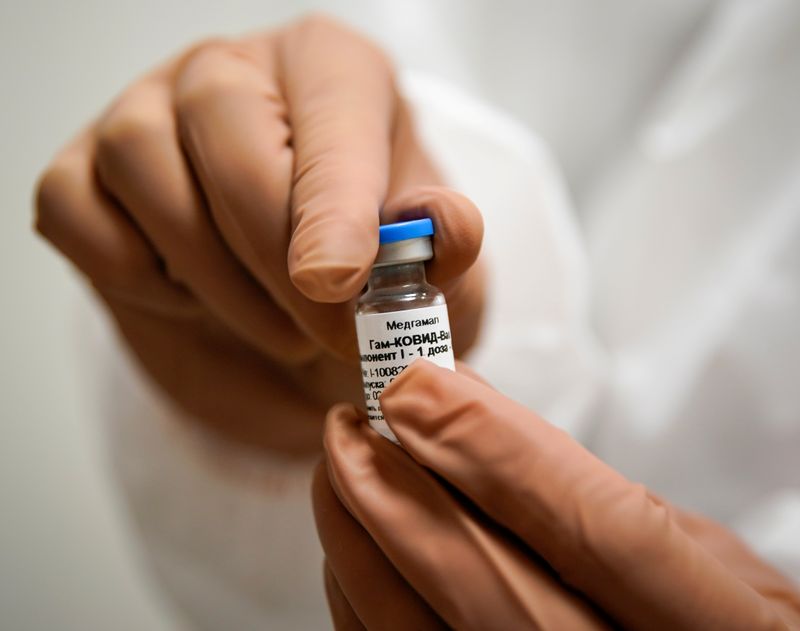
HONG KONG/BEIJING (Reuters) – China’s Tibet Rhodiola Pharmaceutical Holding <600211.SS> announced a deal on Wednesday to manufacture, sell and test Russia’s COVID-19 vaccine in China, hours after interim results showed it was 92% effective at protecting people from the disease.
The initial results of the Sputnik V vaccine are only the second to be published from a late-stage human trial in the global effort to produce vaccine that could halt a pandemic that has killed more than 1.2 million people and ravaged the world economy.
Rhodiola said it plans to conduct early and mid-stage trials of the Russian vaccine in China and final-stage trials overseas, although the trials are yet to be approved by regulators.
Rhodiola, currently unable to produce the Russian vaccine, said it would outsource early development and manufacturing work, and would also consider building production lines at its subsidiary.
The deal requires Rhodiola’s unit to supply the Russian firm with enough vaccine doses to inoculate at least 20 million people in 2021. It adds to several manufacturing deals Russia has announced so far, including a plan to produce 300 million doses in India.
The agreement would also allow Rhodiola to supply vaccine produced in China to designated buyers overseas.
While China has five vaccines in the final stage of clinical trials, local firms have also partnered with foreign drugmakers to test and distribute front-running vaccines in the country.
Shanghai Fosun Pharmaceutical Group <600196.SS> is seeking regulatory approval to trial a product from Pfizer <PFE.N> and BioNTech <22UAy.DE>, and Shenzhen Kangtai Biological Products <300601.SZ> aims to begin human tests for AstraZeneca’s <AZN.L> candidate this year.
(Reporting by Roxanne Liu and Meg Shen, editing by Louise Heavens and Bernadette Baum)