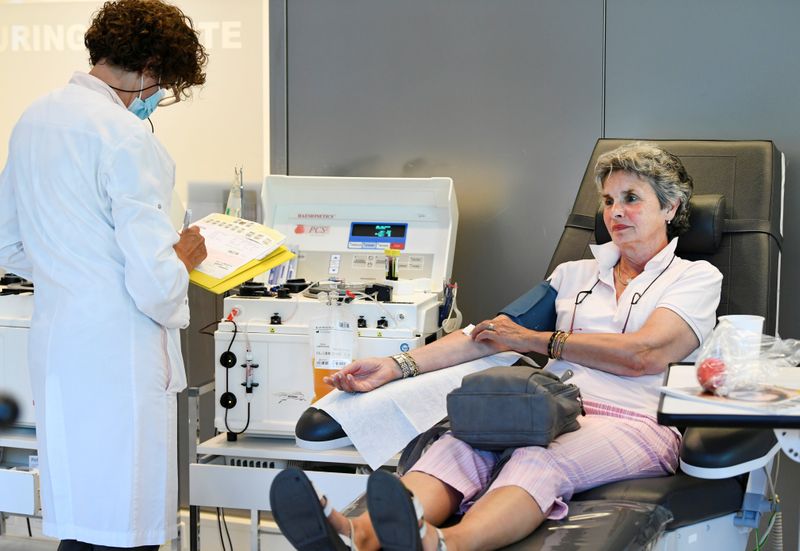
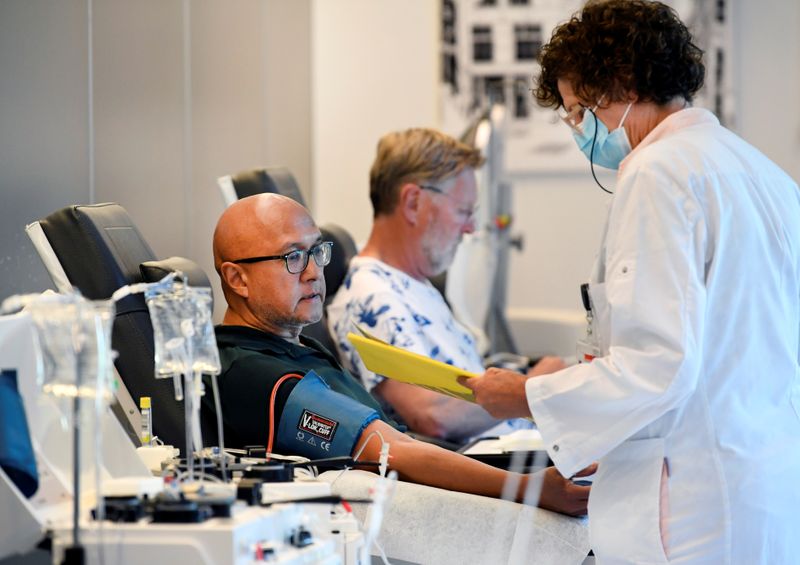
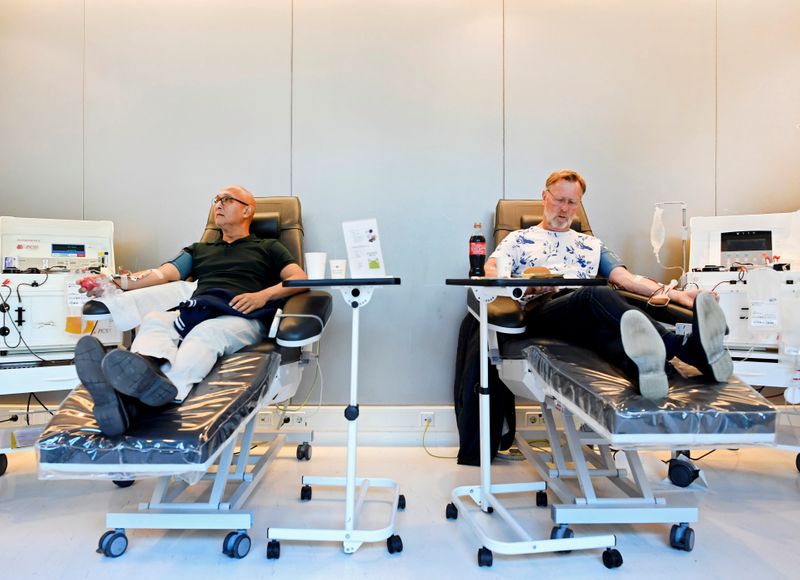
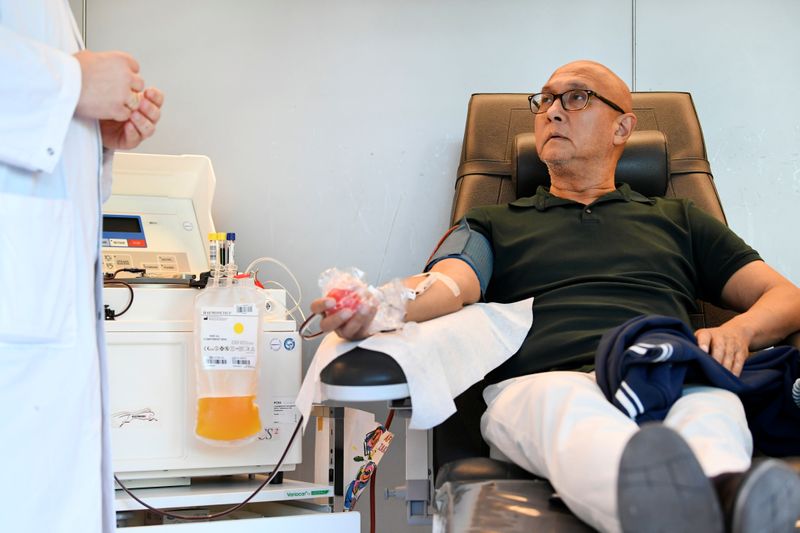
AMSTERDAM (Reuters) – Medical researchers in the Netherlands have signed up 1,500 people who have recovered from the new coronavirus to donate blood as part of an international push to develop a treatment for the virus from their plasma.
Survivors of COVID-19, the disease caused by the new coronavirus, are generally left with blood containing antibodies or proteins made by the body’s immune system to fight off the virus. The blood component that carries the antibodies can be collected and given to newly infected patients.
Doctors are already using survivor plasma to treat coronavirus patients in a number of hospitals worldwide, but plasma supplies are limited because relatively few people have had the new virus and donated blood.
“The whole goal is to pull the resources, put the brightest minds together, to make sure that at the earliest possibility this therapy becomes available,” said Merlijn van Hasselt of blood donation firm Sanquin.
The Dutch group is part of a non-profit alliance, which also includes Japanese pharmaceutical firm Takeda <4502.T>, seeking to collect blood plasma from thousands of donors and purify it into a high-grade treatment that would be approved by medical regulators.
Other alliance members include CSL Behring <CSL.AX> in the United States, Germany’s Biotest AG in Germany, Britain’s BPL Group, LFB SA in France, and Octapharma AG in Switzerland.
“If the clinical trials go well… this might be one of the earliest treatment possibilities for patients,” Van Hasselt said.
Using the blood plasma of survivors of a disease has proven effective in the past in treating people with a range of illnesses including rabies, hepatitis-B and chickenpox.
Separately, Sanquin is testing thousands of samples of donated blood for antibodies to see how far the new coronavirus has spread in the Netherlands.
The first results released in April estimated about 3% of the Dutch population had been exposed.
(Reporting by Esther Verkaik; Writing by Toby Sterling; Editing by Gareth Jones)