(Reuters) – Eli Lilly and Co <LLY.N> said on Thursday it had started mid-stage testing of one of its experimental treatments for COVID-19, as the drugmaker reported second-quarter sales that missed estimates due to the pandemic.
The company has been working on two antibody treatments, LY-CoV555 in partnership with Canadian biotech AbCellera, and another being developed with Chinese drugmaker Shanghai Junshi Biosciences Co Ltd <1877.HK>.
Lilly told Reuters last month it could have a drug specifically to treat COVID-19 and authorized for use as early as September, if either of the two antibodies prove successful in trials.
The company said on Thursday LY-CoV555 had moved to mid-stage trials, while the other treatment had demonstrated safety and tolerability to move into further stages of development.
Lilly’s quarterly sales missed estimates, as it took a nearly $500 million hit due to the COVID-19 pandemic.
Half of that hit came from a delay in patients starting new therapies, Eli Lilly said, and the other half from reduced buying that offset the gain from initial stockpiling by customers during the first quarter.
Lilly, which beat second-quarter profit estimates, also raised its full-year profit forecast.
The company now expects 2020 adjusted earnings between $7.20 and $7.40 per share, from its prior range of $6.70 to $6.90 per share.
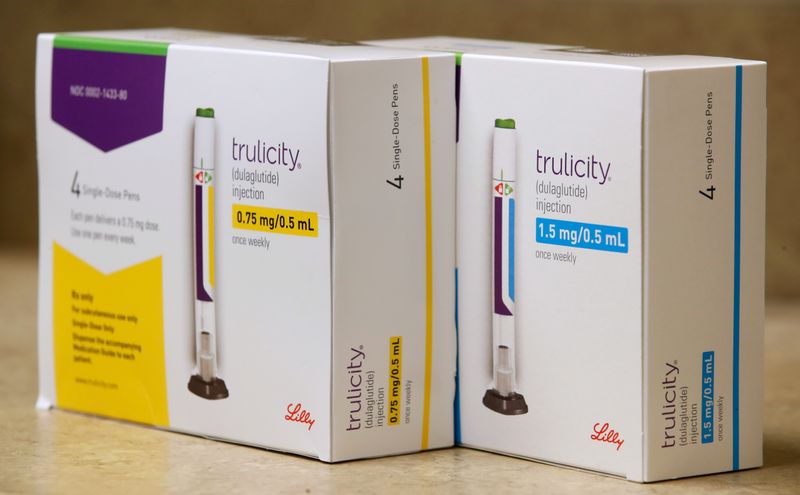
Sales of its diabetes drug Trulicity, the company’s biggest money earner, rose about 20% to $1.23 billion, beating estimates of $1.21 billion, according to five analysts polled by Refinitiv.
On an adjusted basis, Lilly earned $1.89 per share, beating analysts’ estimates of $1.56 per share, according to IBES data from Refinitiv.
Revenue fell 2.4% to $5.50 billion, missing estimates of $5.76 billion.
The company’s shares fell 1.7% before the opening bell.
(Reporting by Manas Mishra in Bengaluru; Editing by Devika Syamnath, Shinjini Ganguli and Shounak Dasgupta)