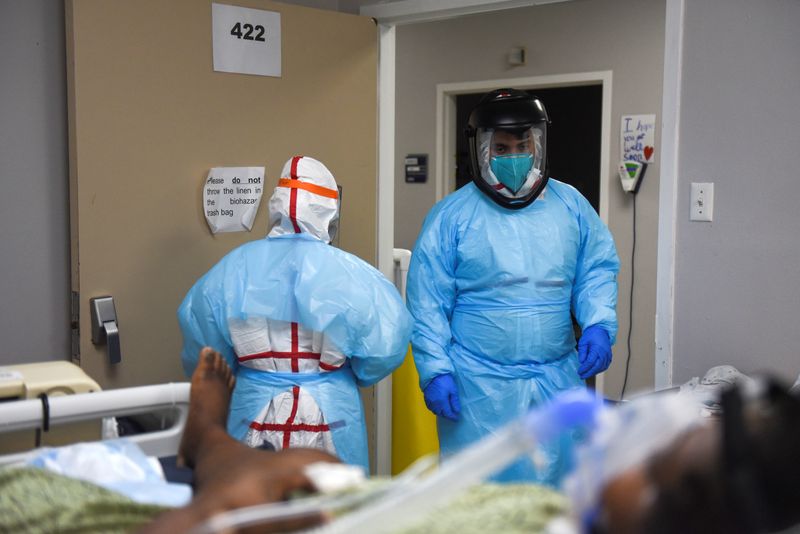
(Reuters) – Dialysis centers in the United States are rolling out COVID-19 antibody treatments this week, a new path for delivery of Eli Lilly and Regeneron drugs approved for emergency use but facing skepticism and logistical problems in some hospitals.
Supplies of the drugs are piling up as hospitals grapple with overflowing wards and mass vaccinations. Kidney dialysis patients are among those most at risk from COVID-19, which is especially deadly among people with chronic illnesses.
Nearly half a million doses of the treatments, manufactured copies of proteins the body produces to combat the virus, have been allocated, but only about 21% of those have been administered, according to the latest statistics from the Department of Health and Human Services.
Fresenius Medical Care, the largest kidney dialysis provider in the United States, said it plans to begin administering the intravenous treatments nationwide this week at facilities dedicated to handling COVID-19 patients or during shifts set up for only those patients.
Smaller dialysis provider U.S. Renal Care said it will begin this week to dispense bamlanivimab, Eli Lilly and Co’s antibody drug, to its patients recently diagnosed with COVID-19.
Timely use of the treatment could help reduce hospital admissions, said Mary Dittrich, the dialysis company’s chief medical officer.
Some doctors have questioned the efficacy of the new treatments, which in any case are complicated to use, taking two hours to infuse and monitor a patient.
“Hospitals aren’t always set up for outpatient infusions. When they are it is typically in environments where compromised patients might be,” said Michael Ganio, senior director of pharmacy practice at the American Society of Health-System Pharmacists.
Nearly 500,000 people in the United States are on dialysis, a process of mechanically cleaning blood usually done three times a week. Diabetes is the most common cause of kidney failure.
Bamlanivimab and Regeneron Pharmaceuticals Inc’s cocktail of casirivimab and imdevimab have the U.S. government’s emergency authorization for non-hospitalized COVID-19 patients who are at high risk of becoming severely ill.
St. John Well Child and Family Center, a non-profit clinic serving low-income families in South and Central Los Angeles, has re-purposed an idled dental office and expects this week to administer the antibody infusion treatments to as many as 18 people a day.
Some hospitals are also opening their own infusion sites separate from vulnerable populations, including cancer patients.
“We have set up an alternate infusion site,” said Dr. William Schaffner, professor of infectious disease at Nashville’s Vanderbilt University Medical Center. “Not everybody wants it. Some say, ‘Gee I am doing pretty well on my own,’ whereas others say, ‘Sure. The president got it, why not me?'”
President Donald Trump was treated with Regeneron’s antibody cocktail, among other drugs, in October after he was diagnosed with COVID-19.
The federal government has multi-million dollar contracts with both Lilly and Regeneron for supplies of the antibody therapies.
U.S. Renal Care said it will initially take delivery of bamlanivimab at 16 clinics in California, Georgia, Hawaii, Maryland, New Mexico, Ohio, South Carolina and Texas.
Vanderbilt’s Schaffner said many hospitals are grappling with a surge of “very sick” patients at the same time they are launching campaigns to inoculate staff with new COVID-19 vaccines.
“The vaccination program is priority one,” he said.
(Reporting By Deena Beasley; editing by Peter Henderson and Grant McCool)