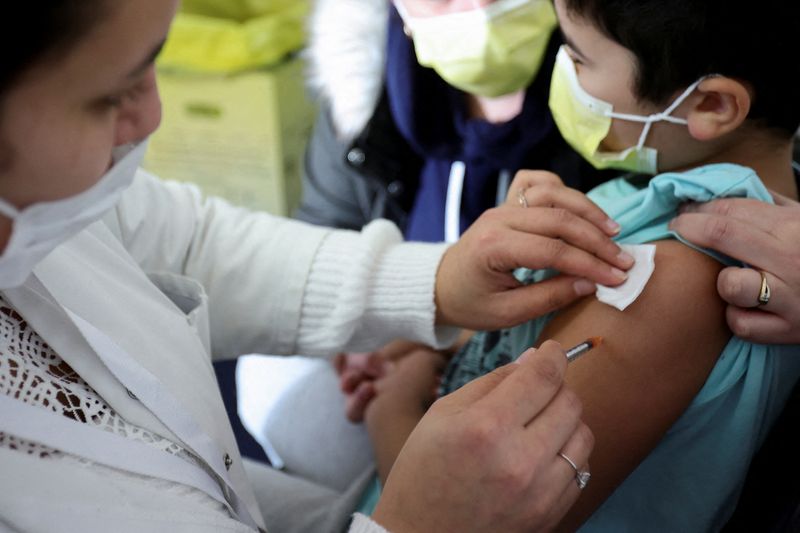
PARIS (Reuters) -France’s Haute Autorite de Sante (HAS) health regulator approved the Pfizer-BioNTech COVID-19 vaccine for all children aged 5-11 on Monday.
The vaccine, which will be administered in a paediatric formulation when it becomes widely available, showed high efficacy among children, said Lise Alter, one of the doctors charged with the risk evaluation of new drugs.
“The HAS suggests that all parents who want it can have their children aged 5 to 11 years vaccinated,” she added.
Last week France started vaccinating 5-11 year olds with medical conditions that require special protection and ramped up logistics to roll out vaccination of all children in the age group once the HAS approves the move.
French President Emmanuel Macron said last week that he was in favour of vaccinating children, but added that this needed to remain the decision of parents.
Alter said that, although COVID-19 rarely caused severe symptoms in children, 80% of severe forms of the disease affected children without pre-existing health conditions.
“With the arrival of the Omicron variant, which is more contagious than the Delta variant, we can expect an increase in severe forms in children without pre-existing health conditions,” she added.
Since the European Medicines Agency (EMA) approved the use of Pfizer-BioNTech’s lower-dose vaccine on the 5-11 age group last month, several countries across the European Union have started vaccinating children in that age group.
(Reporting by Tassilo Hummel; Editing by Sudip Kar-Gupta and Pravin Char)