(Reuters) – It wasn’t easy to build a COVID-19 antibody test during Illinois’ statewide lockdown.
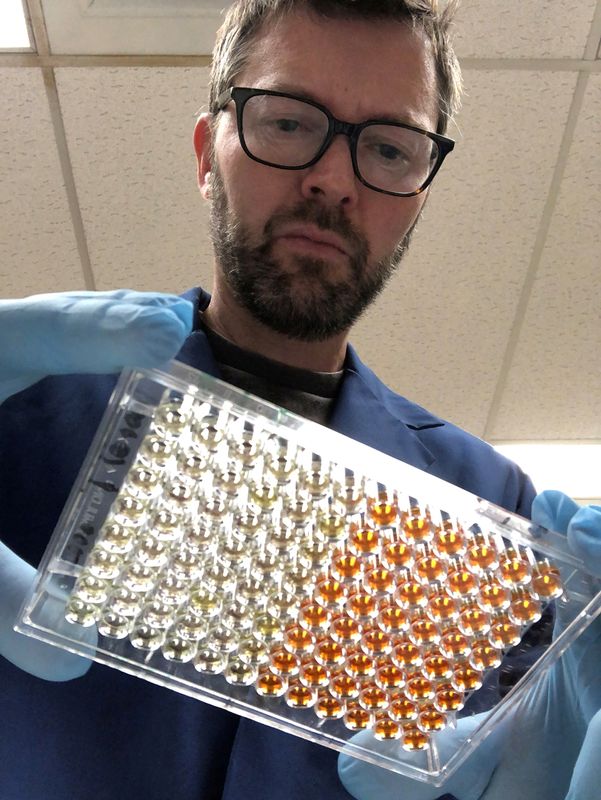
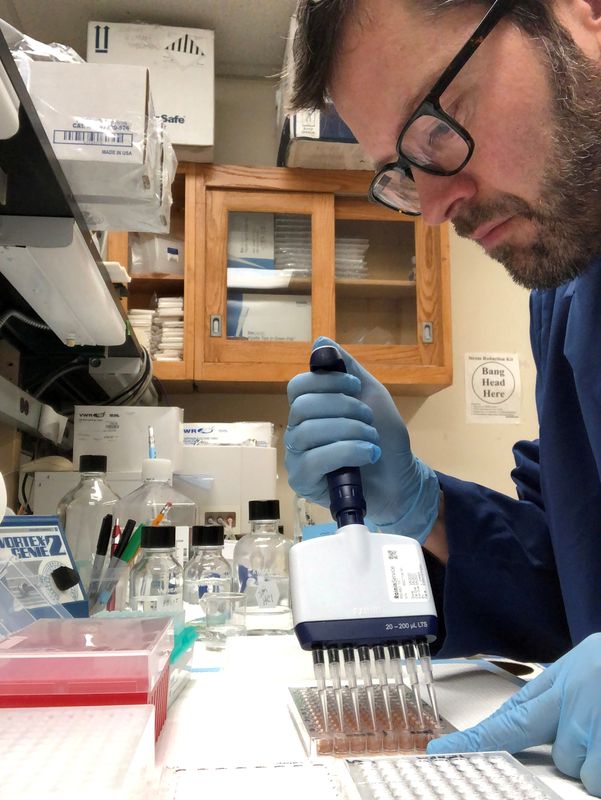
In April, when a key enzyme couldn’t be delivered to his shuttered laboratory, Northwestern University researcher Thomas McDade hunted for the package across the empty campus near Chicago, finally locating it at a loading dock. To verify the test’s accuracy, the biological anthropologist and his colleague, pharmacologist Alexis Demonbreun, asked friends and family if they’d be willing to spot them some blood. McDade took a sample from his wife over their kitchen table.
The scientists’ goal is to look for people with coronavirus antibodies – proteins that indicate possible immunity – in Chicago-area neighborhoods with vastly different death rates. They want to study why the novel coronavirus is deadlier for some groups, such as African Americans and Latinos.
In early May, the team arrived at a test they believed worked. “All the pieces are in place,” McDade wrote in an email to colleagues on May 20. “All we need now is funding to roll it out.”
Across the United States, academic and regional government researchers have embarked on antibody testing initiatives to determine how the coronavirus is spreading, how deadly it is and who is likely to suffer its worst effects. The results could help return the country to normalcy, scientists say, by revealing who is still susceptible and guiding prevention efforts and lockdown decisions..
Unlike clinicians focused mainly on diagnosing individual patients, these scientists study states, communities and other subgroups. But their jobs are made much harder, they say, in the absence of substantial federal funding, standards and coordination.
“It would be better to have a unified information sharing system, a unified testing strategy, and the resources to execute it everywhere in the country,” said Dr. Joe DeRisi, co-president of the Chan Zuckerberg Biohub, a medical research nonprofit in San Francisco.
Instead, the success of coronavirus antibody studies often depends on luck, personal commitment and whatever resources researchers can muster, according to Reuters interviews with more than 20 scientists, public health officials and others.
In a statement, Mia Heck, a spokeswoman for the Department of Health and Human Services, said the administration of President Donald Trump “continues to improve America’s testing capabilities and capacity. As part of these efforts, the Administration is working with diagnostics companies and academic researchers to develop the next generation of tests, testing platforms, and testing protocols. Antibody testing is a particularly important area of ongoing inquiry.”
Of the more than $35 billion allocated by the federal government for COVID-19 testing, relatively little has been earmarked for antibody testing and research. HHS has focused much more on diagnostic testing, which reveals active infections, as opposed to antibody surveillance, which seeks to identify people who have ever been infected with COVID-19, even if they showed no symptoms.
However, $306 million of the federal money has been set aside for the National Cancer Institute to expand antibody testing capacity and fund research projects, according to a public presentation last month by Dr. Dinah Singer, the institute’s deputy director of scientific strategy and development. The institute will award money to private-sector clinical researchers and academic institutions, creating a coordinating center for sharing resources and data.
The U.S. Centers for Disease Control and Prevention, which is overseen by HHS, plans to test for antibodies in 325,000 samples from blood donors in 25 major metropolitan regions over 18 months, beginning this summer.
ALL OVER THE MAP
So far, large-scale blood antibody tests, both by academic researchers and local or state governments, indicate that most people remain vulnerable to coronavirus infection. But results vary – sometimes widely.
Researchers at Massachusetts General Hospital in Boston did one study based on a street corner in a Chelsea, Massachusetts, neighborhood with high COVID-19 hospitalization rates, finding that 31.5% of people had antibodies. In another study across several Boston neighborhoods, the same researchers found 10%.
New York state has tested more than 50,000 people since April, finding antibodies in 12.3% of the population statewide but 20% in New York City, according to Jonah Bruno, a state Department of Health spokesman.
Several antibody studies have been conducted in more affluent areas, where funding was more readily available. Academics from three universities in the San Francisco Bay Area, for instance, have launched at least four antibody blood test initiatives.
In Bolinas, California, a team from the University of California, San Francisco (UCSF), tested more than 1,800 people for coronavirus and its antibodies after locals crowdfunded the cost. The isolated beach community in Marin County, one of the country’s richest, is overwhelmingly white. Diagnostic testing in April showed zero infections, and antibody testing showed between zero and 0.3% had ever been infected.
“I wouldn’t have necessarily chosen Bolinas as the one community to test,” said Dr. Matt Willis, Marin County’s public health officer.
With little standardization, some early efforts at antibody testing have proved inconsistent, onerous and of questionable quality.
A study in April out of Stanford University found that the number of people positive for COVID-19 antibodies in Santa Clara County, California, was more than 50 times the number of infections confirmed through diagnostic testing. Critics derided the results, in part because subjects were recruited via Facebook, potentially skewing participation toward those who had experienced symptoms. The authors argued they had adjusted for selection bias, but nevertheless revised their estimates downward.
Researchers seeking to make their own tests can face difficulty obtaining ingredients like proteins, test plates and color-changing enzymes.
The quasi-public Biodefense and Emerging Infections Resources (BEI), funded by the National Institute of Allergy and Infectious Diseases (NIAID), provides free materials to registered researchers looking to make tests, including those for COVID-19 antibodies. But it’s first come, first served, and some scientists say they have waited weeks for the materials they need.
About 1,300 researchers have applied for registration at BEI since February – more than 90% for COVID-19 research – compared to a normal rate of about 70 applicants per month, a NIAID spokeswoman said. She said that, in general, registration takes three days and materials are shipped out within another three.
Even with the proper materials in hand, scientists say it can be difficult to compare one researcher’s results with another’s.
“If I measure antibodies in my lab, using a method I cobbled together from available sources, and someone else cobbles theirs together with a different suite of sources, we’re going to get different results,” said Eleanor Brindle, a researcher at the University of Washington who is studying whether nursing mothers can pass the virus or its antibodies to their infants.
GOOD FORTUNE AND INGENUITY
McDade got lucky. He was able to join forces with Northwestern pharmacologist Demonbreun, who had a key ingredient on hand and helped him quickly assemble a blood antibody test.
The university helped pay for the initial work, and the team has applied for more funding. Decisions like that can take a while, given the competition for research dollars and the challenges posed by COVID-19.
“These days, when we’re wrestling with issues about how to bring research back to campus, how to plan for a fall quarter that brings students back to campus, we’ve really been swamped,” said Milan Mrksich, Northwestern’s vice president of research.
The team sought support from several other potential funders, so far without success. But last month, they secured a $200,000 grant from the National Science Foundation.
Even that wasn’t easy: The NSF initially told McDade the project was not a good fit, he said. He tweaked the proposal in May, focusing it on potential scientific innovations. That brought “the work more in line with NSF’s mission,” said agency spokesman Michael England, making it worth the investment.
McDade said the NSF money is enough to test 3,000 people – less than a third of the goal – but he hopes it can help leverage more funding. He just wishes the federal government would open the spigots more widely.
“Why are academics like me trying to find little pots of money to do what we think is a basic public health surveillance effort?” he said. “It’s ludicrous.”
(Nick Brown reported from New York; Editing by Michele Gershberg and Julie Marquis)