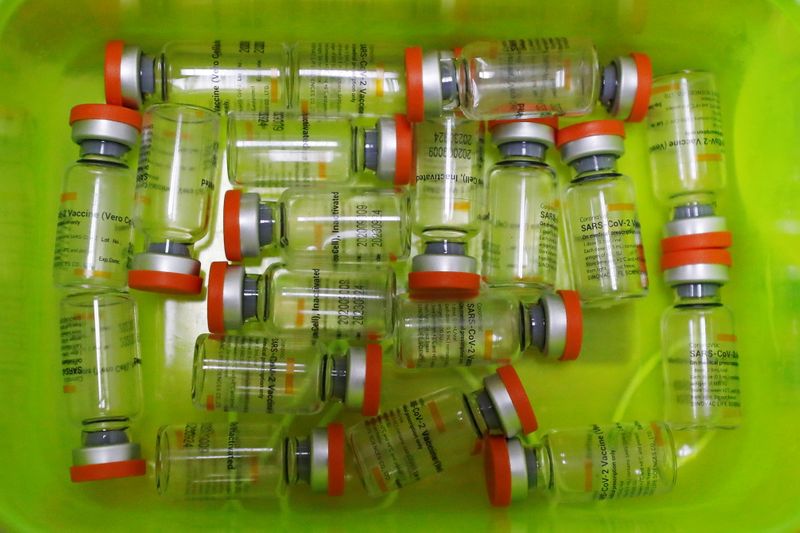
HONG KONG (Reuters) – Hong Kong has formally approved China’s Sinovac vaccine for emergency use with the rollout starting on Feb. 26, the city’s health secretary said on Thursday, paving the way for residents in the global financial hub to be vaccinated for COVID-19.
Sophia Chan said the vaccine met the “safety, efficacy and quality requirements specified in Hong Kong emergency situations” and that the benefits outweighed the risks.
Patrick Nip, secretary for civil service, said the government expected to receive a million doses of Sinovac vaccines on Friday afternoon with vaccinations expected to start on Feb. 26.
Chan, who was speaking at a news briefing together with Nip, addressed what she called “doubts” about vaccine safety and said all vaccines authorised by the government have been assessed to be safe by experts.
“The vaccines give us hope of returning to normal lives.”
A Hong Kong government advisory panel on COVID-19 vaccines said on Tuesday it recommended Sinovac vaccine for emergency use.
The recommendation came after the government exempted Sinovac from publishing results of its third phase clinical trials in medical journals due to the “urgency” for vaccination.
The BioNTech vaccine – the first vaccine approved by Hong Kong’s Health department – was required to have results published in a medical journal before being examined by the advisory panel on COVID-19 vaccines.
Nip said the BioNTech vaccine would arrive in Hong Kong before the end of February.
Residents will be able to get vaccinated at 29 centres across the city with five offering Sinovac and 24 offering BioNtech vaccines. Priority will be given to health workers, over 60s and those working in cross-border transportation.
(Reporting by Donny Kwok, Justin Chan and Anne Marie Roantree; Writing by Farah Master; Editing by Christopher Cushing)