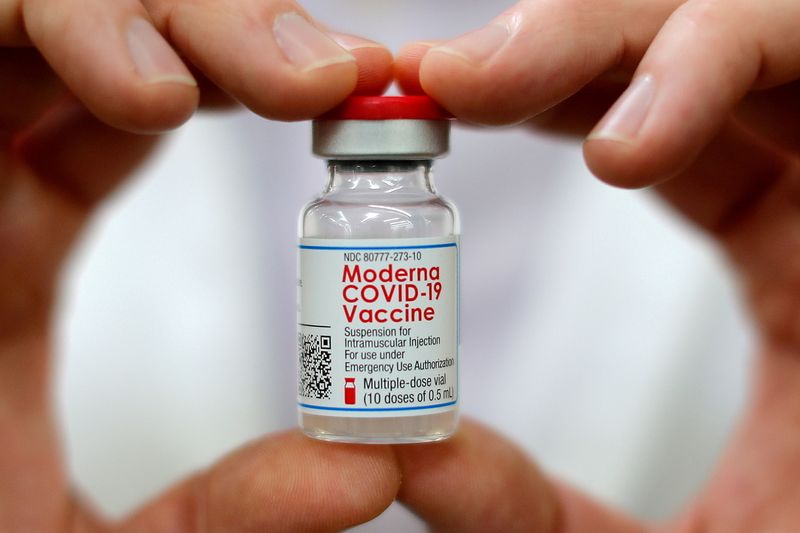
(Reuters) -Moderna Inc said on Wednesday it had applied with the U.S. Food and Drug Administration for authorization of its COVID-19 booster vaccine for all adults aged 18 and older.
The FDA has cleared booster shots of COVID-19 vaccines for people who are immunocompromised, those aged 65 and older and for individuals at high risk of severe disease or who are regularly exposed to the virus.
Moderna is seeking authorization for a 50-microgram booster dose, half the strength of its original vaccine given in two shots about four weeks apart.
The filing comes a week after Pfizer Inc applied for a similar clearance for the booster doses of the vaccine it has developed with German partner BioNTech.
A decision from the FDA on Pfizer is expected this week ahead of a U.S. Centers for Disease Control and Prevention (CDC) advisory panel meeting on Friday to discuss expanding the eligibility for booster doses of the vaccine.
Moderna’s shares rose 5% to $246.16 in afternoon trading on the news.
Experts believe booster doses are vital for reducing COVID-19 to an endemic level and U.S. President Joe Biden’s administration in August announced plans to roll out booster doses for all adults in September.
Last month, the European Union’s drug regulator approved Moderna’s booster vaccine for all age groups over 18, at least six months after the second dose. The company has also applied for booster approval in Japan.
However, regulators in several countries, including France, Canada, Finland and Sweden, have taken a more defensive stance on Moderna’s vaccine over heart-related safety concerns affecting younger people.
(Reporting by Manas Mishra and Mrinalika Roy in Bengaluru; Editing by Sriraj Kalluvila)