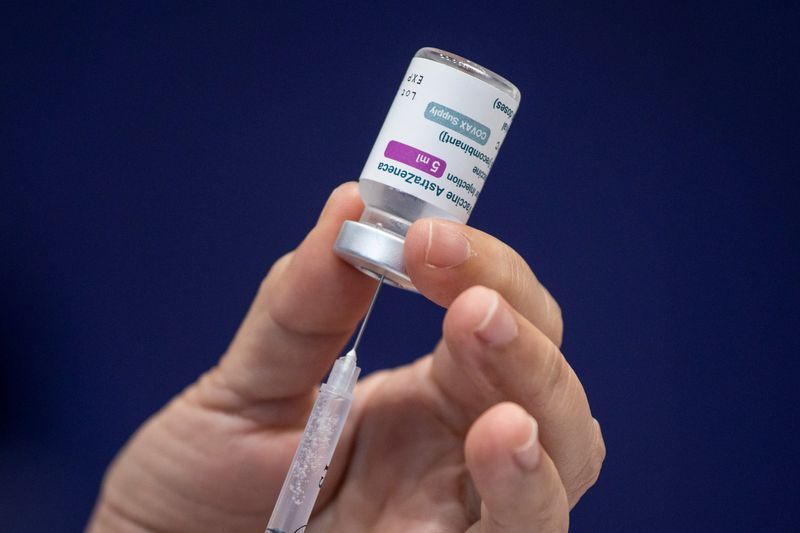
MANILA (Reuters) – Philippine health authorities suspended on Thursday the use of AstraZeneca’s COVID-19 vaccine for people below 60 years of age to investigate reports of blood clots coming from overseas.
The temporary suspension came after the European Medicines Agency recommended to include blood clots as a rare side effect of the AstraZeneca vaccine, Food and Drug Administration chief Rolando Enrique Domingo said in a statement, adding that there were no reports of such adverse side effects in the country.
“This temporary suspension does not mean that the vaccine is unsafe or ineffective – it just means that we are taking precautionary measures to ensure the safety of every Filipino,” Domingo said.
The Southeast Asian country, which is battling one of the worst coronavirus outbreaks in Asia, has been counting on speeding up a sluggish rollout of vaccinations to help alleviate pressure on hospitals and boost its pandemic-battered economy.
The Philippines has so far received 525,600 doses of AstraZeneca vaccines, about a fifth of the country’s total inventory, through the COVAX facility. Another 2.6 million doses, purchased by the private sector, will be delivered next month.
The Philippines kicked off its inoculation programme on March 1, starting with healthcare workers. Vaccinations have since expanded to people with co-morbidities and the elderly.
It has so far administered nearly 923,000 doses of China’s Sinovac Biotech and AstraZeneca vaccines, part of its goal of inoculating 70 million of its 108 million population this year.
(Reporting by Neil Jerome Morales; Editing by Ed Davies)