By Nathan Allen and Joan Faus
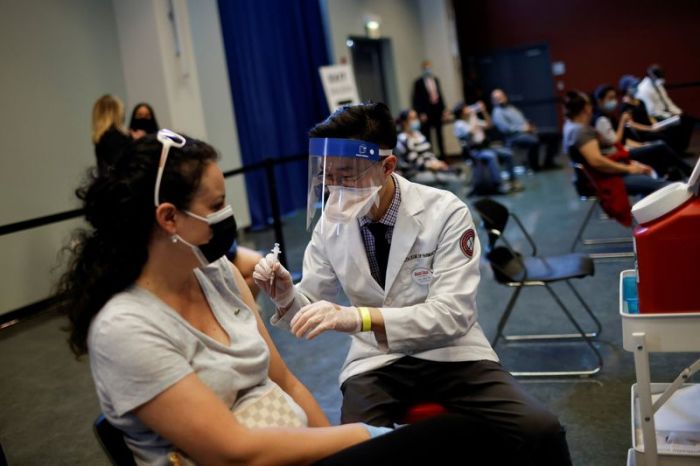
MADRID (Reuters) -Spain’s health ministry said on Tuesday it was not aware of any changes to the planned first delivery of 300,000 Johnson & Johnson COVID-19 shots expected to arrive on Wednesday.
J&J said on Tuesday it would delay the rollout of its COVID-19 vaccine in Europe and was reviewing cases of extremely rare blood clots in people after they received the shot with European health authorities.
U.S. federal health agencies on Tuesday recommended pausing the use of the vaccine as six women under 50 developed rare blood clots after receiving the shot, dealing a fresh setback to efforts to tackle the pandemic.
The U.S. announcement triggered a 6% drop in the share price of Spanish pharmaceutical company Reig Jofre which will fill and bottle J&J’s vaccine from mid-June at its Barcelona plant.
Reig Jofre declined to comment.
The company is awaiting further information but it currently maintains its production calendar, a source close to Reig Jofre said.
Spanish Prime Minister Pedro Sanchez said on Tuesday the benefits of all the approved COVID-19 vaccines outweighed risks.
Asked about the U.S. recommendation at a news conference, Sanchez said he was not aware of the suspension but stressed that all vaccines approved for use in Europe met safety criteria.
(Reporting by Inti Landauro, Nathan Allen and Joan Faus, editing by Andrei Khalip and Nick Macfie)