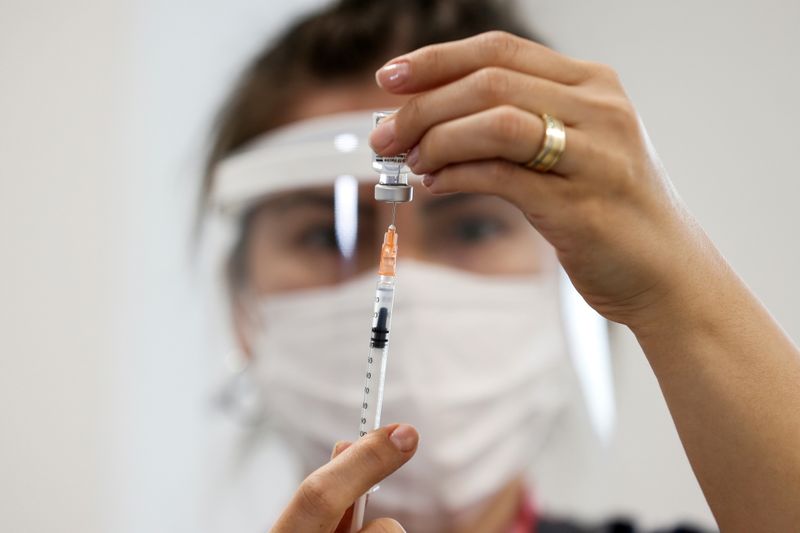
ISTANBUL (Reuters) – Turkey on Tuesday kicked off Phase III trials for its COVID-19 vaccine, named “Turkovac” by President Tayyip Erdogan who said the shot should be available for use by the end of the year.
Ankara currently administers vaccines developed by Sinovac, as well as by Pfizer and Biontech. Turkey also granted an emergency use authorization for Russia’s Sputnik V. People over 25 are eligible for the vaccine.
“It is not clear how long this disease will go on and how much mutation it will go through. It is critical that we have our own vaccine against this disease,” Erdogan told an event to mark the inoculation of the first Phase III volunteer with the vaccine, which uses an inactivated virus.
Turkey has sharply accelerated COVID-19 vaccinations, delivering more than 1 million a day last week and prompting hopes of a rebound in the economy and tourism sector.
(Reporting by Ezgi Erkoyun; Editing by Dominic Evans)