NEW YORK (Reuters) – U.S. health insurers may balk at covering tests that look for coronavirus antibodies in some cases, arguing that employers or the government should foot a bill expected to run into billions of dollars.
Health insurers have largely escaped the economic pain wrought by the pandemic. Their profits increased as many Americans delayed more routine and expensive medical care during the recent lockdown period, while the total cost of covering COVID-19 patients has been less than expected in many regions with low case numbers.
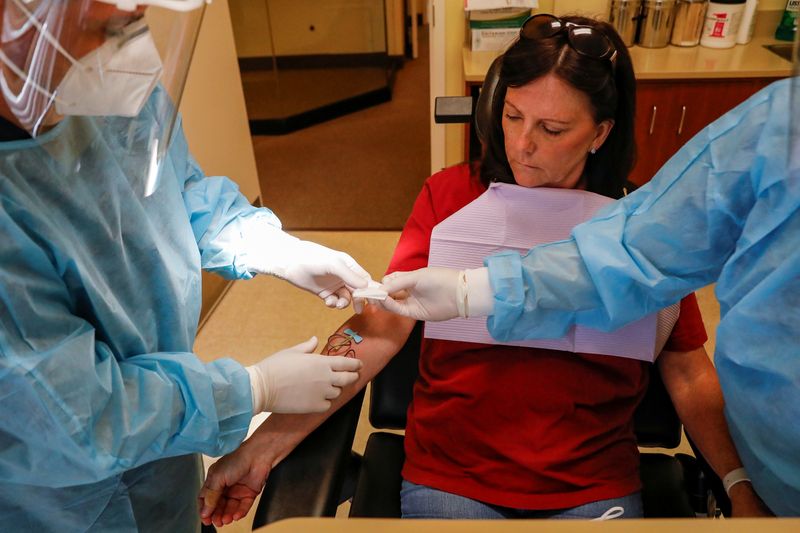
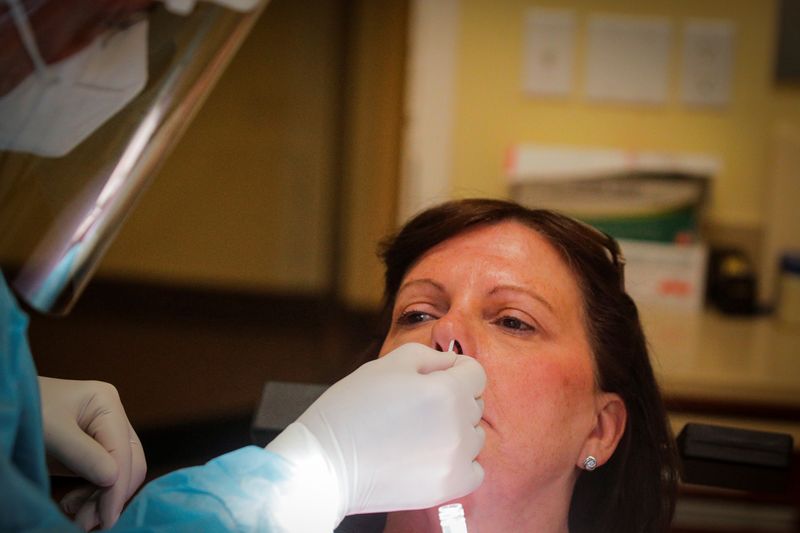
Now the industry is tallying up the potential cost of expanding both diagnostic and antibody testing, seen as a critically important component of safely reopening businesses across the country. Diagnostic tests determine if someone is currently infected and contagious, while the antibody, or serology, tests show whether someone was previously infected and possibly immune.
Wall Street firm Jefferies & Co estimates a need for hundreds of millions of antibody tests in the next 18 months, accounting for about one-quarter of an anticipated $15 billion in U.S. COVID-19 test spending through the end of 2021.
In pushing back, health insurers draw lines between what they deem as medically necessary tests, and those done for research or return-to-work purposes.
Businesses may require repeat testing of employees, and they note that private health insurance covers only about half of Americans. The government needs to provide guidance on the role of insurers, employers and public health officials, insurers say.
“It is also critical that these strategies consider related funding in that context,” Kristine Grow, spokeswoman for America’s Health Insurance Plans said in a statement. AHIP estimates antibody testing will cost the United States as much as $19 billion a year.
The biggest national insurers including UnitedHealth Group Inc, CVS Health Corp’s Aetna unit and Cigna Corp have policies to cover the tests, which cost around $50, and related appointment fees, when doctors say they are medically necessary. Others, such as Blue Cross of Idaho, say they will not cover the tests to help determine the prevalence of the disease in a community, which officials see as necessary for controlling subsequent outbreaks.
Doctors’ groups and diagnostics makers say broad serology testing is an important component of understanding COVID-19 and the level and length of immunity prior infection confers.
AdvaMedDX, a trade group that represents test makers including Roche Holding AG and Abbott Laboratories, expects about 94 million laboratory-based tests to be shipped by the end of June. It has sent letters to Blue Cross of Idaho and Blue Cross & Blue Shield of Mississippi telling them their policies do not align with the law. Neither company responded to a request for comment.
“Clinicians really need at their disposal the full range of COVID-19 testing,” said Susan Van Meter, executive director of AdvaMedDX.
The U.S. Congress passed legislation calling for health insurance coverage for COVID-19 testing to cope with a pandemic that has killed more than 115,000 people and infected over 2 million nationwide. The federal government set aside $26 billion at the beginning of May for coronavirus testing, much of which was earmarked for government health agencies, states and research and development.
The U.S. Centers for Disease Control and Prevention has since instructed Americans not to assume a positive antibody test means they are immune from future infection, and to continue physical distancing and use of masks until there is more evidence around immunity.
LIMITING USE
Covering only tests insurers see as medically necessary could limit them to people who either have symptoms of COVID-19 or been in close contact with someone who has. They may also cover testing for recovered COVID-19 patients who want to donate antibody-rich plasma for treatment of others or for children with a rare coronavirus-related complication.
The uncertainty around coverage of tests for other purposes is slowing a return to business for Andrew Matta, who runs the North American Dental Group.
The group has 200 affiliated locations in about 15 states, including New York, Georgia and Texas, with more than 1 million patients. It is working with a physician’s group that orders both a diagnostic and serology test ahead of dental procedures. The patient then has a blood draw at the dentist’s office, and returns three days later for the procedure.
Dental patients are starting to return with basic safety protocols in place, but Matta expects testing to help reassure customers more wary of coronavirus exposure.
Insurers have been noncommittal about whether they will cover the cost of that testing, or whether patients will end up being billed by the testing lab, he said.
“If we knew these would be covered for pre-procedures, we would be scaling it rapidly right now,” Matta said.
Sabrina Corlette, a health policy professor at Georgetown University, had been advocating for private insurers to pay for these tests broadly. She now says there should be a separate government-run fund for testing to make sure patients – insured or uninsured – can easily get the tests they need.
“If people fear they are going to face hundreds of dollars in bills, they are not going to get tested,” Corlette said, “and it’s a risk not just to them, but to everybody around them.”
(Reporting by Caroline Humer; editing by Michele Gershberg and Bill Berkrot)